New material could revolutionize solar fuel generation
27. 7. 2018 | FAPESP | www.fapesp.br/en
Following the isolation of graphene in 2004, a race began to synthesize new two-dimensional materials. 2D materials are single-layer substances with a thickness of between one atom and a few nanometers (billionths of a meter). They have unique properties linked to their reduced dimensionality and play a key role in the development of nanotechnology and nanoengineering.
An international group of researchers including Brazilian scientists affiliated with the University of Campinas (UNICAMP) have succeeded in producing a new material with these characteristics. The researchers extracted a 2D material they call hematene from ordinary iron ore like that mined in many parts of the world, including Brazil. The material is only three atoms thick and is thought to have enhanced photocatalytic properties.
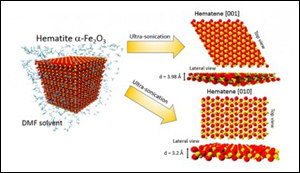
The new material was exfoliated from hematite, one of the most common minerals on earth and the main source of iron, which is the cheapest metal, used in many products and above all to make steel. Unlike carbon and its 2D form graphene, hematite is a non-van der Waals material, meaning it is held together by 3D bonding networks rather than by nonchemical and comparatively weaker atomic van der Waals interactions, which are noncovalent.
Read more at FAPESP
Image Credit: FAPESP
-jk-




