New type of electrolyte could enhance supercapacitor performance
19. 8. 2019 | MIT | www.mit.edu
Supercapacitors, electrical devices that store and release energy, need a layer of electrolyte — an electrically conductive material that can be solid, liquid, or somewhere in between. Now, researchers at MIT and several other institutions have developed a novel class of liquids that may open up new possibilities for improving the efficiency and stability of such devices while reducing their flammability.
For decades, researchers have been aware of a class of materials known as ionic liquids — essentially, liquid salts — but this team has now added to these liquids a compound that is similar to a surfactant, like those used to disperse oil spills. With the addition of this material, the ionic liquids “have very new and strange properties,” including becoming highly viscous, says MIT postdoc Xianwen Mao PhD ’14, the lead author of the paper. “This proof-of-concept work represents a new paradigm for electrochemical energy storage. ”
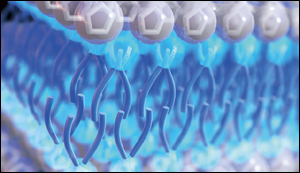
The key to its effectiveness is the way the molecules within the liquid automatically line themselves up, ending up in a layered configuration on the metal electrode surface. The molecules, which have a kind of tail on one end, line up with the heads facing outward toward the electrode or away from it, and the tails all cluster in the middle, forming a kind of sandwich. This is described as a self-assembled nanostructure.
Read more at MIT
Image Credit: Xianwen Mao, MIT
-jk-




