New Material May Help Cut Battery Costs for Electric Cars
7. 7. 2017 | University of Texas at Dallas | www.utdallas.edu
In the battle of the batteries, lithium-ion technology is the reigning champion, powering that cellphone in your pocket as well as an increasing number of electric vehicles on the road.
But a novel manganese and sodium-ion-based material developed at The University of Texas at Dallas, in collaboration with Seoul National University, might become a contender, offering a potentially lower-cost, more ecofriendly option to fuel next-generation devices and electric cars.
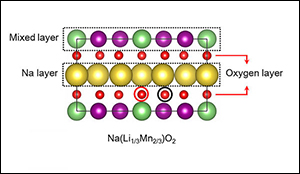
The research team's sodium-ion design, which retains the high energy density of a lithium-ion cathode, replaces most of the lithium atoms (green) with sodium (yellow). The layered structure of the new material also incorporates manganese (purple) and oxygen (red).
A battery consists of a positive electrode, or cathode; a negative electrode, or anode; and an electrolyte in between. In a standard lithium-ion battery, the cathode is made of lithium, cobalt, nickel and oxygen, while the anode is made of graphite, a type of carbon. When the battery charges, lithium ions move through the electrolyte to the anode and attach to the carbon. During discharge, the lithium ions move back to the cathode and provide electric energy to run devices.
Read more at University of Texas at Dallas
Image Credit: University of Texas at Dallas
-jk-




