Chemically Storing Solar Power
22. 2. 2016 | TU Wien | www.tuwien.ac.at
By combining highly specialised new materials, the scientists at TU Wien (Vienna) have managed to combine high temperature photovoltaics with an electrochemical cell. Ultraviolet light can be directly used to pump oxygen ions through a solid oxide electrolyte. The energy of the UV light is stored chemically.
The key to success was an unusual choice of materials. Instead of the ordinary silicon based photovoltaics, special metal oxides - so-called perovskites - were used. By combining several different metal oxides, the scientists managed to assemble a cell which combines photovoltaics and electrochemistry.
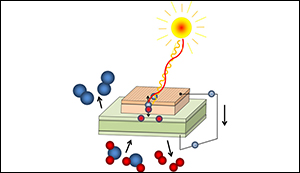
New cell consists of two different parts – a photoelectric part on top and an electrochemical part below. In the upper layer, ultraviolet light creates free charge carriers, just like in a standard solar cell. The electrons in this layer are immediately removed and travel to the bottom layer of the electrochemical cell. Once there, these electrons are used to ionize oxygen to negative oxygen ions, which can then travel through a membrane in the electrochemical part of the cell.
Read more at TU Wien
Image Credit: TU Wien
-jk-




